02
-
Introduction
- Week 2
Objective This week we will be finishing off the ideas of the periodic table and extending this to explain the different ways in which atoms can join together- a process we call BONDING Study 1. Read over week 2 lecture presentation.
2. Read chapter 2.5-2.6, 2.8 in Callister
3. Work through the questions on page 17 of the study pack and other relevant questions at the end of the chapter in Callister.Activity Have a look at these web sites: periodic table and Crash course chemistry
-
1.2 Periodic Table and Bonding
- All the elements have been classified according to electron configuration in the periodic table Here, the elements are situated, with increasing atomic number, in seven horizontal rows called periods. The arrangement is such that all elements that are arrayed in a given column or group have similar valence electrons structures, as well as chemical and physical properties.
Fig. 1.3 shows the arrangement of electrons into shells for the first few elements. Since the number of protons in the nucleus governs the total number of electrons in all shells around it, it follows that there are generally insufficient electrons to complete the final outer shell.
At the same time, there is electrical attraction between the outer shell electrons of one atom and the nuclear protons of neighbouring atoms and it is these forces of attraction which cause both chemical combination and physical changes such as crystallisation to take place. As a result of such reactions strong bonds are formed between atoms so that the atoms are left with completed outer electron shells. This is achieved by atoms either losing, gaining or sharing electrons. - All the elements have been classified according to electron configuration in the periodic table Here, the elements are situated, with increasing atomic number, in seven horizontal rows called periods. The arrangement is such that all elements that are arrayed in a given column or group have similar valence electrons structures, as well as chemical and physical properties.
-
The Ionic Bond
- This bond is produced by the transfer of electrons between atoms, usually between metal and non-metal atoms. For example, the extremely reactive metal sodium, combines with the equally reactive non-metallic gas chlorine, to form crystals of sodium chloride (common table salt).
An atom of sodium contains eleven protons in its nucleus and, therefore, eleven electrons in orbit around that nucleus. Two of these electrons complete the first shell, eight in the second shell, leaving a lone electron in the third shell. The atom of chlorine on the other hand contains seventeen protons in its nucleus and, consequently, seventeen electrons in obit around it. Two of these fill the first shell, eight in the second, so that there are no less than seven electrons in the third shell.
Since the nuclear charge of the sodium atom is relatively small its attraction for the lone electron in the outer orbit is weak and so, if a sodium atom and a chlorine atom approach each other, the lone electron of the sodium atom is snatched away so that it joins the outer electron of the chlorine atom. This leaves the sodium particle with a complete second shell (8 electrons) and at the same time the electron which it has lost goes to complete the third shell (8 electrons) of the chlorine particle. Because the sodium particle has lost a negatively- charged electron it now has a resultant positive charge; whilst the chlorine particle having gained an electron has a resultant negative charge. Figure 3 shows the formation of the ionic bond between sodium and chlorine.
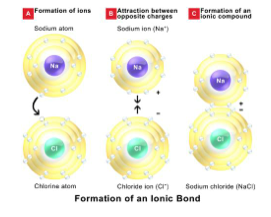
- Figure 3: The formation of the ionic bond in sodium chloride, by the transfer of an electron from the sodium to the chlorine atom.
- These charge particles are no longer simply atoms - the sodium particle is now less than an atom whilst the chlorine particle is more than an atom. Charged particles of this type called ions. Metals always form positively charged ions whilst non-metals form negatively charged ions. As these sodium and chlorine ions carry opposite charges they will attract each other. However, whilst unlike charges attract, like charges repel so that sodium and chlorine ions arrange themselves in a geometrical pattern which each chlorine ion is surrounded by six sodium ions as its nearest neighbours whilst each sodium ion is surrounded by six chlorine ions (Figure 4).
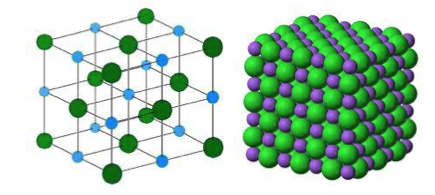
- Figure 4 A simple cubic crystal lattice such as exists in solid sodium chloride.
- This bond is produced by the transfer of electrons between atoms, usually between metal and non-metal atoms. For example, the extremely reactive metal sodium, combines with the equally reactive non-metallic gas chlorine, to form crystals of sodium chloride (common table salt).
-
The Metallic Bond
- Most metals have one, two or at most, three electrons in the outermost shell of the atom. These outer-shell electrons are loosely held to the atomic nucleus and as a metallic vapour condenses and subsequently solidifies these outer-shell electrons are surrendered to a sort of common pool and are virtually shared between all atoms in the solid metal. Since the resultant metallic ions are all positively charged they will repel each other and so arrange themselves in some form of regular (crystal) pattern in which they are firmly held in position by the attractive force between them and this permeating 'cloud' of negatively charged electrons (Figure 5).
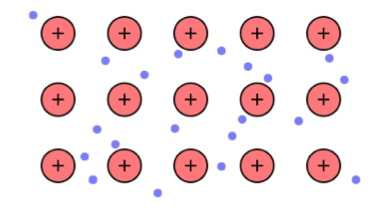
- Figure 5: Diagrammatic representation of the metallic bond (sea-of–electrons model)
1. All metals are good conductors of electricity
What we call a 'current of electricity' is in fact a stream of moving electrons. Since electrons constituting the 'electron cloud' are free to move within the body of the metal it follows that a metal conducts electricity. If we force in some electrons at one end of a metal wire (by using a head of electrons or an electrical potential difference, as it is termed), we can take out an equal number of electrons at the other end, providing we give them another conductor into which they can escape.2. Metals are good conductors of heat
The application of heat to a piece of metal causes electrons to vibrate more actively and these vibrations can be passed on quickly from one electron to another within the electron cloud.3. Most metals are ductile because layers of ions can be made to slide over each other by the application of a shearing force.
At the same time metals are strong because the attractive force provided by the electron cloud opposes the sipping apart of those layers of ions4. Metals are lustrous in appearance since the free, vibrating surface electrons reflect back units of light as these fall upon the surface of the metal. -
The Covalent Bond
- The covalent bond is formed between atoms of those non-metallic elements in which for various reasons, there is a strong attractive force between the nucleus and the outer-shell electrons. Hence instead of a transfer of electrons form one atom to the other there is a sharing of electrons between two atoms thus binding them together.
The hydrogen atom is the most simple of all atoms. It consists of a single electron in orbit around a nucleus consisting of a single proton. Two atoms of hydrogen will therefore combine to form a molecule of hydrogen and in this way the two electrons are shared between the two atoms thus completing the first electron shell for each atom by this process of sharing the two electrons (Figure 6). Thus a mass of hydrogen gas does not contain single atoms but rather a conglomerate of rapidly moving diatomic (containing two atoms) molecules.
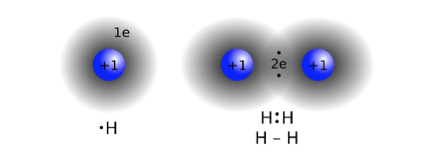
- Figure 6: Formation of the Hydrogen molecule
- Similarly an atom of carbon which has four electrons in its outer shell (the second electron shell) can combine with four atoms of hydrogen. The resultant unit containing a tightly bound association of one carbon atom with four hydrogen atoms constitutes a molecule of the gas methane, the principal component of the natural gas obtained currently from the North Sea bed and the most simple of all the many thousands carbon-hydrogen compounds or organic compounds as they are called which are derived from living matter whether plant or animal. In the methane molecule (Figure 7) the carbon atom shares four electrons contributed by the four hydrogen atoms and so the outer shell of eight electrons is completed. Similarly each hydrogen atom shares one of the electrons provided by the carbon atom and so its outer shell of two is completed. The methane molecule is thus a stable unit.
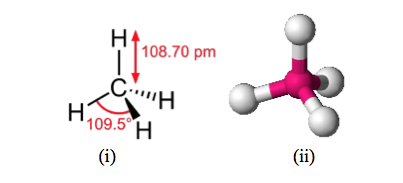
- Figure 7:
i) Covalent bonding in a molecule of methane CH4
ii) Spatial arrangement of atoms in the methane molecule.
- The element carbon has the ability to form long chain-like molecules in which carbon atoms are covalently bonded to each other and to hydrogen atoms.
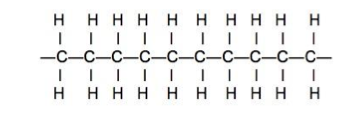
- Figure 8: Polyethylene molecule (© Carnegie Mellon Uni.)
- Figure 8 shows a molecule of the material polyethylene, better known as polythene being formed from many molecules of ethylene. By using chemical means to replace some of the hydrogen atoms by atoms, or groups of atoms, of other elements, a wide variety of plastics materials can be produced.
An important feature of these materials is that, unlike metals in which the outer-shell electrons can travel freely within the electron cloud so making metals conductors of electricity, the outer-shell electrons in these covalent substances are securely held to the atoms to which they belong. These electrons are not free to move away and so the materials are excellent insulators since they cannot conduct electricity. - The covalent bond is formed between atoms of those non-metallic elements in which for various reasons, there is a strong attractive force between the nucleus and the outer-shell electrons. Hence instead of a transfer of electrons form one atom to the other there is a sharing of electrons between two atoms thus binding them together.
-
Inter Molecular Forces
- However large the molecules in covalent compounds they will always contain equal numbers of protons and electrons contributed by their constituent atoms. Therefore, they will be electrically neutral and carry no resultant charges. So why do these molecules stick together to form a coherent solid mass?
The short answer is that electrons and protons are not necessarily equally distributed over the molecule, often due to interaction with electrons and protons of neighbouring molecules. Consequently, due to this irregular distribution of electrons and protons the molecule acquires what is sometimes called an electric dipole moment, that is it has a 'negative end' and a 'positive end' rather like the north and south poles of a magnet (Figure 9).
Since unlike charges attract (as do the unlike poles of magnets) then molecules will be attracted to each other. The greater the unevenness of distribution of the charges within molecules the greater the dipole moments and forces of attraction between them and therefore the 'stronger' the material. Uneven distribution of electrons within a molecule may occur for a number of reasons and will give rise to intermolecular forces of different strengths. These small forces of attraction are known collectively as van der Waal's forces. In the case of small molecules the total van der Waal's forces operating between large molecules like those of polythene the sum total of all of the van der Waal's forces operating between a molecule and its neighbour is great and the solid so formed is relatively strong.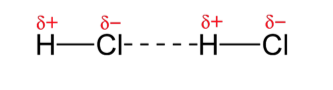
- Figure 9(a): The principle of the dipole moment.
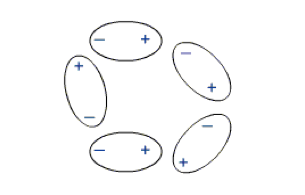
- Figure 9(b) Van Der Waal bonding
- Video Support
- Periodic Table
- Bonding
- However large the molecules in covalent compounds they will always contain equal numbers of protons and electrons contributed by their constituent atoms. Therefore, they will be electrically neutral and carry no resultant charges. So why do these molecules stick together to form a coherent solid mass?
-
ATOMIC STRUCTURE AND BONDING TUTORIAL
- 1. Describe the basic structure of the atom using the Bohr model
2. Explain how the periodic table can be used to describe the properties of the elements.
3. Discuss each of the possible primary and secondary bonding types in terms of the requirements and resultant effects.
4. Describe the the theory used to explain both thermal and electrical conduction in metals. (sea-of-electron model) - 1. Describe the basic structure of the atom using the Bohr model