07
-
What is thermodynamics?
- Thermodynamics is the science which investigates the relationship between the physical and chemical properties of materials i.e. working substance, temperature, pressure and volume when it exposed to different processes (expansion, compression, cooling or heating)
Many engineering systems involve working fluids undergoing various transformations of phases in order to convert one form of energy to another for the desired application. We encounter such systems in every day of our life, for examples; electrical power plants, vehicle engine, turbo-jet engine, refrigerator, air-conditioning systems, international space station etc. In all these we are concerned with the conversion of heat and work.
The name thermodynamics stems from the Greek word thermotis (heat) and dynamiki (potential, power).
Thermodynamics can be defined as the science, which deals with the conversion of heat to power in relation to the properties of the system.
Formally the study of thermodynamics began in the 19th century as a consequence of the need to understand and develop a system that produce work using hot objects, known as heat engine.
- Thermodynamics Animation 📹
- Thermodynamics is the science which investigates the relationship between the physical and chemical properties of materials i.e. working substance, temperature, pressure and volume when it exposed to different processes (expansion, compression, cooling or heating)
-
Thermodynamic System
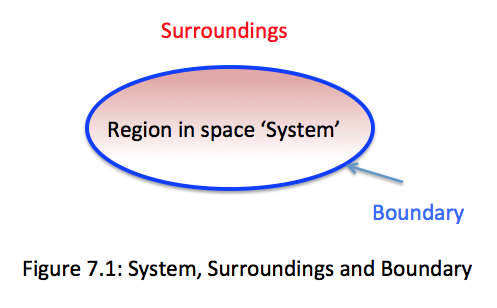
- A thermodynamic system is defined as a quantity of matter or a region in space with fixed or movable boundaries chosen for study. For defining the object under study, we draw a boundary around what we wish to study.
The mass or region outside the system is called the surroundings. The surface that separates the system from its surroundings is called boundary. Boundary: The real or imaginary surface that separates the system from its surroundings. It can be fixed or movable and mathematically, the boundary has zero thickness, no mass, and no volume.
Surroundings: Everything outside the boundary that interacts with the system is called surroundings
The interactions between a system and its surroundings can happen through one of the following three categories: heat, or work or transfer of mass.- Thermodynamic System 📹
- System may be as simple as a melting ice cube or as complex as nuclear power station.
Examples of thermodynamic systems:
⎯ Internal Combustion Engine 📹
⎯ Refrigerator
⎯ Turbojet Engine 📹
⎯ Power plant
⎯ Space station
⎯ Wind Turbine 📹
⎯ Slider-crank mechanism 📹

7.2.1 Closed System
- A closed system is one where there is no flow of fluid into or out of the region under consideration. It therefore follows that a closed system can transfer heat and work across a boundary, but not mass.
- Example of a Closed System 📹
- Let us consider the piston-cylinder arrangements shown in Figure.7.2 and illustrated in the above Piston Cylinder link.
Since we are focusing our attention on the gas, hence it becomes our system and the boundary is the inner surfaces of the piston and the cylinder. As it has been observed in the slide animation no mass crosses the boundary of the system and hence the system is classified as a closed system. When energy crosses the boundary in the form of heat we notice that part of the boundary (the inner surface of the piston, in this case) start to move. Everything outside the gas, including the piston and the cylinder, is the surroundings.
7.2.2 Open System
- An Open System is a region in space properly selected for investigation. In open system both mass and energy can cross the boundary of the system. It is often called a control volume.
- Open System (Control Volume) 📹
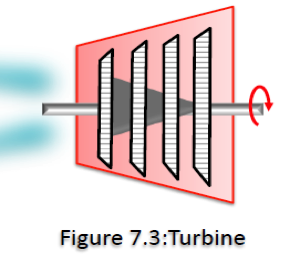
- As an example of an open system, consider the turbine shown in Figure 7.3 and illustrated in the above Open System link,
Such turbines are used in power generation plants. In these applications energy carried by the working fluid (gas or steam) entering the turbine and expands to a lower pressure as power is generated. The working fluid exists at a lower pressure and temperature. It is not convenient to choose a fixed mass as our system for the analysis. Instead, we can concentrate our attention on the volume formed by the interior surface of the turbine and consider the hot and cooler working fluid streams as mass entering and leaving the control volume. The interior surface of the turbine form the control volume for this case, and mass is crossing the control volume at two locations. - A thermodynamic system is defined as a quantity of matter or a region in space with fixed or movable boundaries chosen for study. For defining the object under study, we draw a boundary around what we wish to study.
-
Approaches to Thermodynamic Systems
- There are two approaches to studying system behaviour. One is called the classical approach or macroscopic approach which will be used in this course of study. Matter will be assumed to be continuous substances, and, when the behaviour of a small element or particle of fluid is studied, it will be assumed that it contains so many molecules that it can be treated as part of this continuum. Quantities such as velocity and pressure can be considered to be constant at any point, and changes due to molecular motion may be ignored. Variations in such quantities can also be assumed to take place smoothly, from point to point.
The other is called statistical approach based on statistical consideration and probability theory that deals with models of behaviour of atoms/molecules making up the system under consideration.
- Thermodynamic Definitions 📷
- Microscopic and Macroscopic approach to Thermodynamics 📹
- There are two approaches to studying system behaviour. One is called the classical approach or macroscopic approach which will be used in this course of study. Matter will be assumed to be continuous substances, and, when the behaviour of a small element or particle of fluid is studied, it will be assumed that it contains so many molecules that it can be treated as part of this continuum. Quantities such as velocity and pressure can be considered to be constant at any point, and changes due to molecular motion may be ignored. Variations in such quantities can also be assumed to take place smoothly, from point to point.
-
Properties and State of a Substance
- Any macroscopic characteristic of matter, which can be measured or monitored, in general, is called property. Examples: mass, volume, density, energy, pressure and temperature. However a thermodynamic property is defined as any quantity that independent of the path (prior history) of the process and only depends on the initial and the final state of the system
- Thermodynamic property can be divided into two general classes: intensive and extensive
Intensive properties: those which are independent of the size (mass) of a system, such as temperature, pressure, and density. They are not additives. Pressure and temperature are two of the intensive properties and, as such, are independent of the size of the system. A cup of tea at 90°C has exactly the same temperature as a pot of tea at 90°C. Both pressure and temperature are independent of the mass of fluid contained within the system.
Extensive properties: values those are dependent on size of the system such as mass, volume, and total energy. Extensive properties per unit mass are called specific properties, e.g. specific volume (v = V/m).
- Properties of a System 📷
- State: The condition of a system as described by its properties
The state of the system is determined by the values of its properties. Two such properties, namely pressure and temperature are particularly relevant as they can be measured directly and can, therefore be visualised, if not as a concept, at least as a position on the graduated scale of a pressure gauge or thermometer.
Other properties that can be measured directly are the volume, mass and velocity of the fluid within the system. In the case of the pressure, temperature and velocity, the property can change with time so that any particular value must be defined at a particular instant. It follows that the state of a system also changes with time as the system undergoes a process.
- State and Process 📹
- However, the velocity is a different type of property to either pressure or temperature. A fluid can have a particular temperature irrespective of whether it has a velocity or not. Velocity is considered to be a mechanical property because it defines the kinetic energy within the system. Another mechanical property is the height of a system within a gravitational field, since this defines the potential energy of the system.
On the other hand, volume does depend on the mass of the fluid. A mass of 2 kg will have twice the volume of a mass of 1 kg, providing the fluid is at the same pressure and temperature. A particular mass has its own unique values of those thermodynamic properties that depend on the quantity of fluid. Volume is one such thermodynamic property.
Other thermodynamic properties are internal energy, enthalpy and entropy.
To overcome the inconvenience of relating these properties to a particular mass of fluid, the values can be quoted in “specific” terms. Namely, the value of the property with relation to a unit mass of the fluid. In the case of specific volume, the units are m3/kg.
Similarly, values of specific internal energy u (kJ/kg), specific enthalpy h (kJ/kg), specific entropy s (kJ/kgK) and Specific volume v (m3/kg) are all quoted with respect to a kg.
A study of thermodynamics is based upon the assumption that the state of a system can be defined in terms of just two thermodynamic properties, providing those two are independent of each other. For example, in the case of a gas, pressure and temperature are independent of each other. Defining a particular pressure and a particular temperature defines the specific volume, which, in turn, defines the other specific properties.
On the other hand, a boiling liquid has a temperature that depends on the pressure. In this case the pressure and temperature are not independent, so that the state of the fluid would need to be defined in terms of either pressure or temperature, and one other independent thermodynamic property.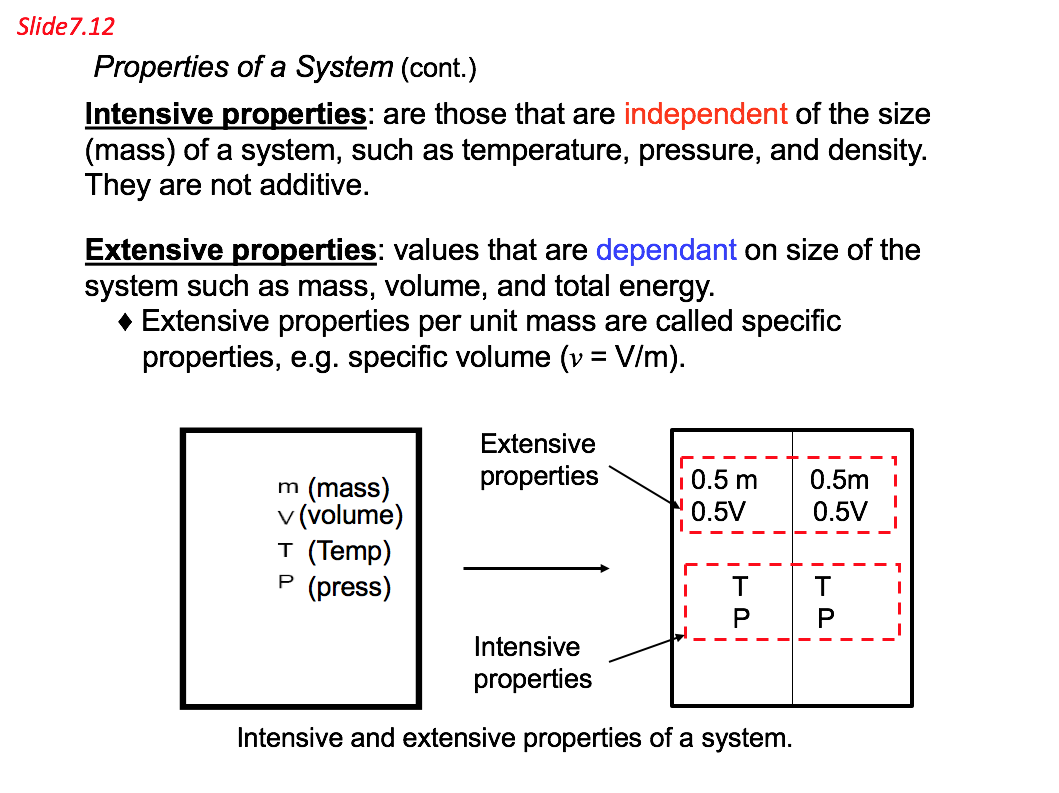
-
State and Equilibrium
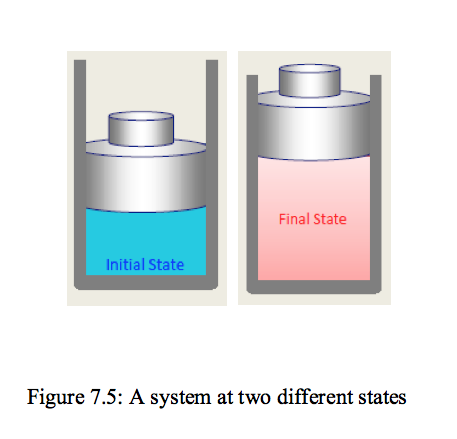
- Consider a system not undergoing any change.
At this point all the properties can be measured or calculated throughout the entire system, which gives us a set of properties, that completely describes the condition, or the state, of the system. At a given state, all the properties of the system have fixed values. If the value of even one property changes, the state will change to different one. In Figure.7.5 a system is shown at two different states.
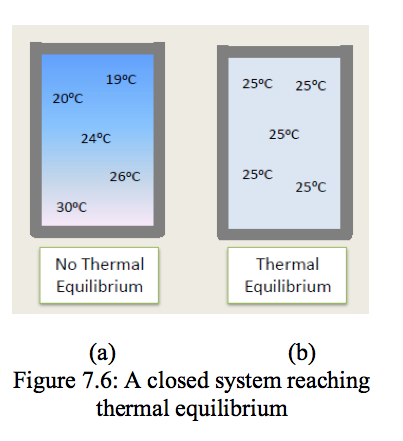
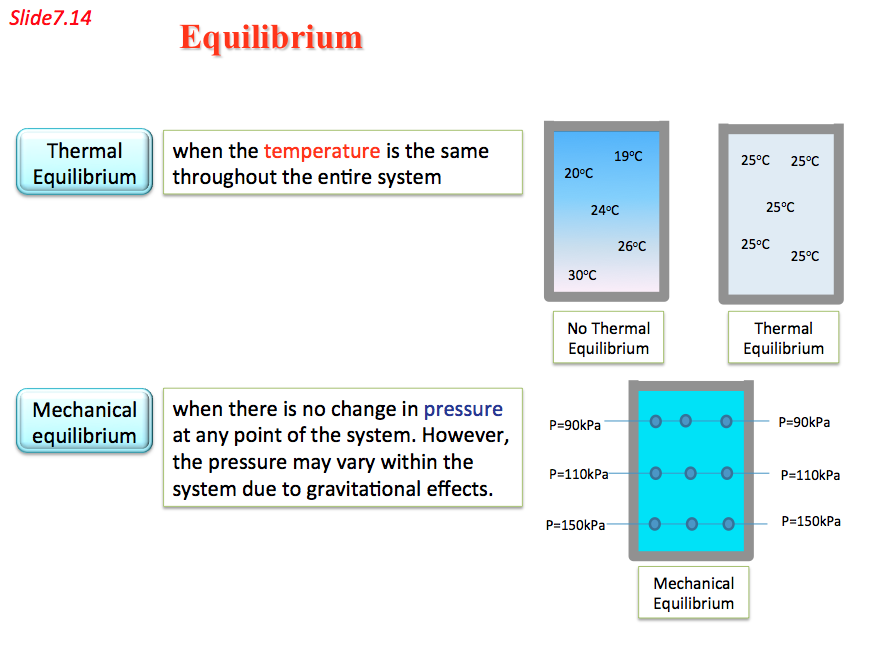
- Thermodynamic deals with equilibrium states. The word equilibrium implies a state of balance. In an equilibrium state there are no unbalanced potentials (or driving forces) within the system. A system in equilibrium experiences no change when it is isolated from its surroundings.
There are many types of equilibrium, and a system is not in thermodynamic equilibrium unless the conditions of all the relevant types of equilibrium are satisfied.
- Equilibrium 📷
- The first of these is thermal equilibrium in which the temperature is constant. Figure 7.6 shows a closed system. In Figure 7.6(a) the fluid is not in thermal equilibrium as there are temperature differences. These temperature differences could give rise to heat transfer within the fluid and to motion of the fluid within the system. In Figure 7.6(b) the fluid has achieved thermal equilibrium conditions, as the temperature is constant throughout the system.
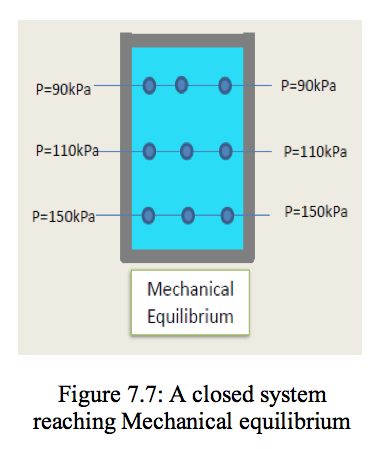
- Similarly, the fluid must have constant pressure throughout the system (Figure 7.7) otherwise the forces within the fluid would be out of balance and the system would not have mechanical equilibrium. Finally, the fluid must have chemical equilibrium so that its chemical composition does not change with time. Therefore, equilibrium is an essential assumption when modelling thermodynamic situations.
- Consider a system not undergoing any change.
-
Thermodynamic Equilibrium and Quasi-Equilibrium Processes
- A quasi-equilibrium or reversible process is one in which the system remains infinitesimally close to equilibrium at all times. This is an idealization that is approximated by many real processes.
Let us assume we have a gas in cylinder-piston assembly initially in equilibrium at state 1. Now let us change the state by compressing or expanding it and allowing the system to settle to reach equilibrium at state 2. The process can be represented on a p-v diagram as shown in Figure 7.8.
States 1 and 2 are in equilibrium and have well-defined properties (e.g. temperature and volume) and hence the laws of thermodynamics can be applied to relate these end states but not any of the intermediate states.
Thus a line representing the path cannot be located on the coordinate diagram (p-v diagram) because the properties such as pressures and densities do not have single unique values throughout the process. However, if the system is imagined to pass through a continuous series of equilibrium states during the process, the intermediate states could be located on the diagram, and a line representing the path of the process could be drawn through all the points. Such a process is called a reversible process or quasi-static process.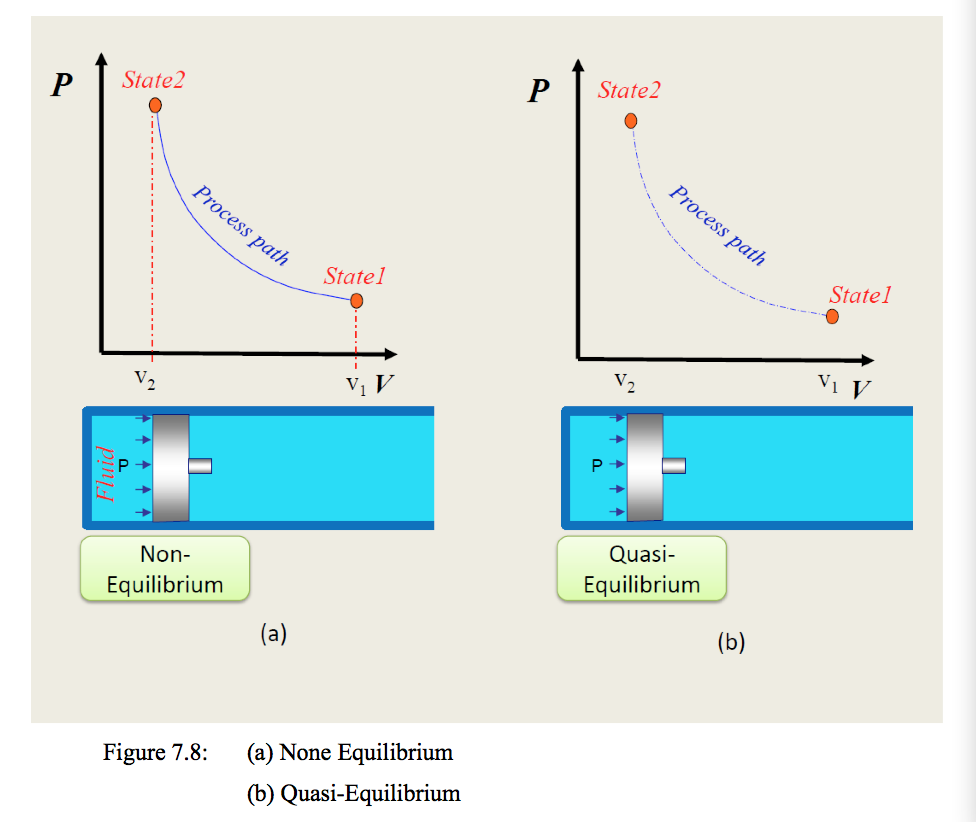
- Equilibrium Animation 📹
- A quasi-equilibrium or reversible process is one in which the system remains infinitesimally close to equilibrium at all times. This is an idealization that is approximated by many real processes.
-
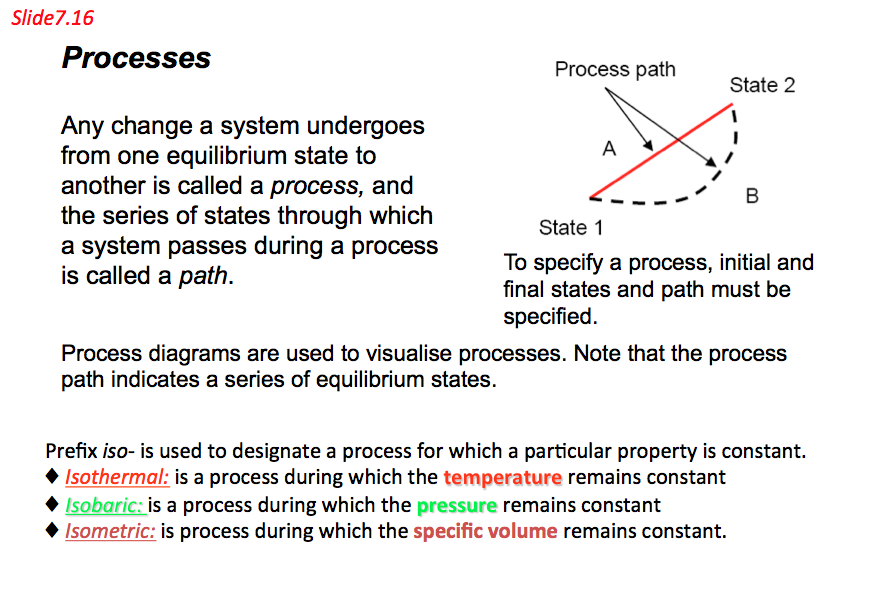
Process and Cycles
- Process: Any change that a system undergoes from one equilibrium state to another is called a process, and the series of states through which the system passes during a process is called the path of the process (Figure 7.9). To describe a process completely, one should specify the initial and the final states of the process, as well as the path it follows, and the interaction with the surroundings.
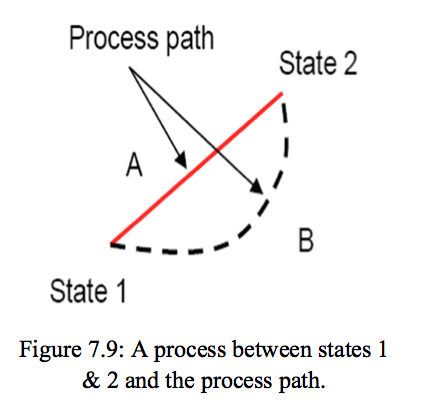
- Processes 📷
- Cycles: When a fluid undergoes a series of different processes and returns to its initial state it is said to have undergone a “cycle” or, more precisely, a thermodynamic cycle. The concept of the cycle is employed in all power generation cycles such as the Rankine cycle shown in Figure 7.10. The various Power cycles will be studied during the second and third year of your programme of study.
- Cycle and Cyclic Process 📹