08
-
8.1 Temperature and Scales
- Our sense of perceptions are used to gauge the strength of force in terms “strong” or “weak” and the temperature in terms of “hotness” or “coldness” of a body. The degree of “hotness” or “coldness” vary from one person to another and hence human are unable to precisely gauge this quality and assign numerical values to temperature based on our sensation. To quantify the temperature accurately some materials with reproducible and predictable change of properties was utilised, for example the expansion of mercury with temperature.
There are two scales commonly used for measuring the temperature, namely; the Fahrenheit scale (named after Gabriel Fahrenheit 1686 - 1736) and the Celsius (named after Anders Celsius 1701 - 1744) scale. The Fahrenheit scale is used with the English Engineering System of Units and the Celsius scale with the System International (SI). Both systems are based on the freezing and boiling points of water which usually called ice point and the steam point respectively. On the Fahrenheit scale these two points are assigned 32 and 212, respectively and on the Celsius scale the two points are 0 and 100 respectively. Symbol oF is used to denote Fahrenheit and symbol oC is used to denote Celsius.
The temperature scale that will be adapted in this course of study is the Celsius scale. However students should learn how to convert from one scale to another. The conversion between the two systems is given by the following equation:
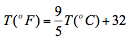
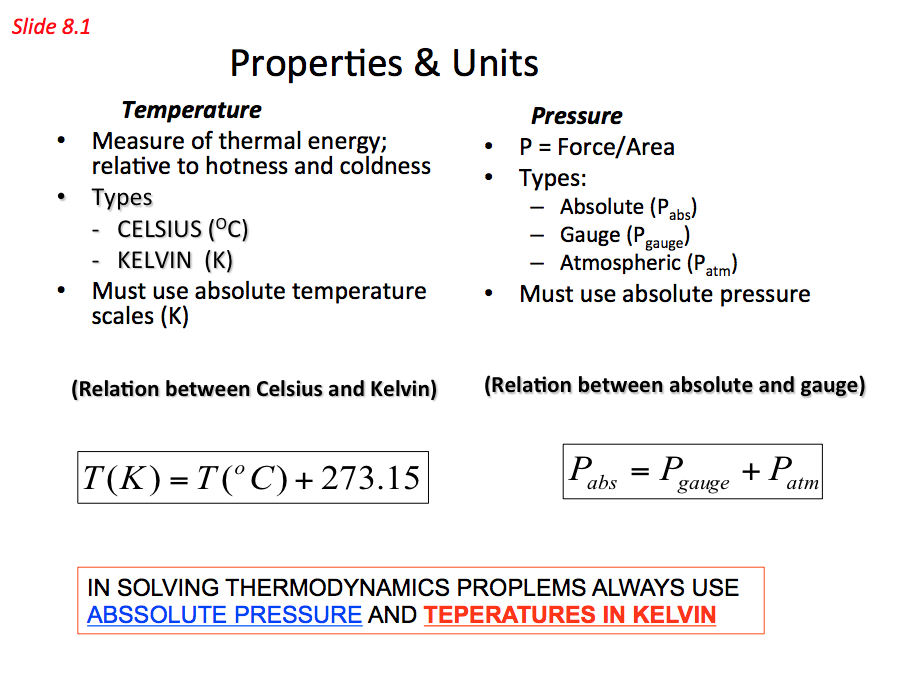
In thermodynamic relationships we use the absolute scale and normally referred to as the thermodynamic scale of temperature and is independent of any thermometric substance can be defined. The absolute scale relate to the Celsius scale is the Kelvin scale named after William Thomson (known as lord Kelvin 1824 – 1907) who was born in Belfast then moved to Glasgow where he attended university from the age of 10. The relation between these scales is: T(K)=(oC)+273.15
VERY IMPORTANT: In thermodynamic relationships, temperature is always in terms of Kelvin scale unless specifically stated otherwise. - Our sense of perceptions are used to gauge the strength of force in terms “strong” or “weak” and the temperature in terms of “hotness” or “coldness” of a body. The degree of “hotness” or “coldness” vary from one person to another and hence human are unable to precisely gauge this quality and assign numerical values to temperature based on our sensation. To quantify the temperature accurately some materials with reproducible and predictable change of properties was utilised, for example the expansion of mercury with temperature.
-
8.2 Pressure in Thermodynamics and Units
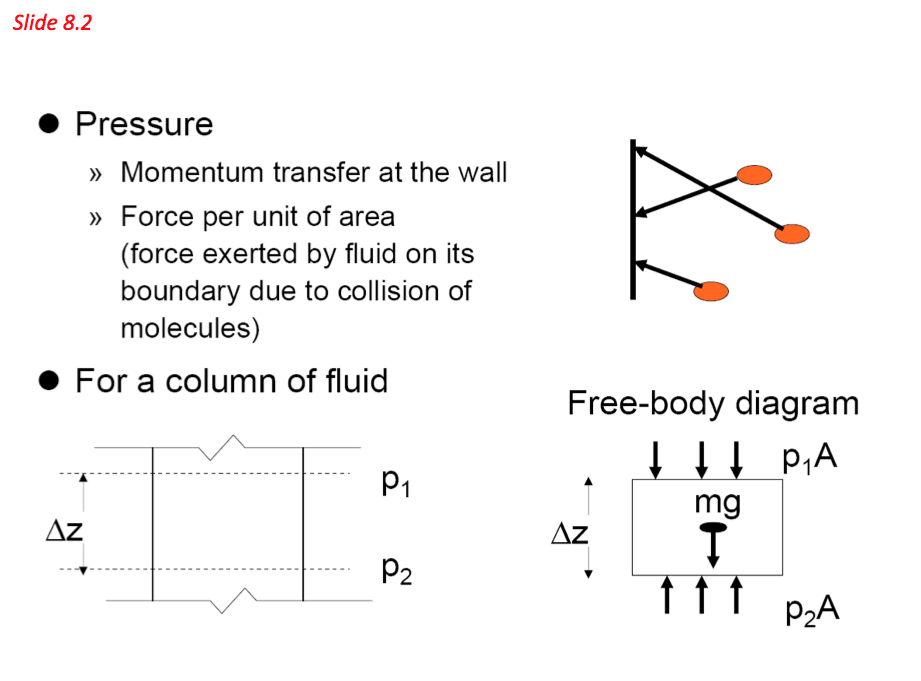
- Pressure is defined as the amount of perpendicular force (F) exerted by a fluid on a unit surface area (A). The term pressure is used for fluid and normal stress for solids. As stated in the definition above, pressure is force per unit area:
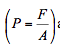
and hence the unit of pressure is newton per square meter (N/m2), which normally known as a Pascal (Pa).
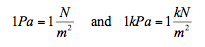
There are other units of pressure such as bar and standard atmosphere (atm)
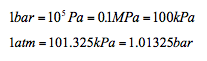
- Properties and Units 📷
8.2.1 Pressure Measurement
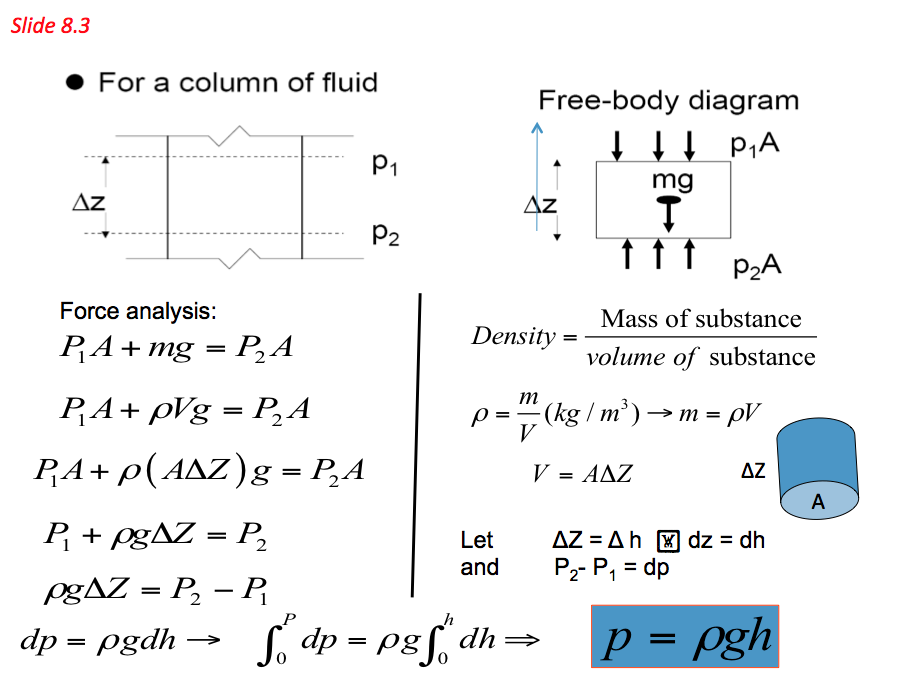
- For a fluid of constant density (ρ) contained in a vertical vessel of cross-section area (A) m2 to a depth (h) m, the mass of the fluid M (kg) can be calculated as follows:
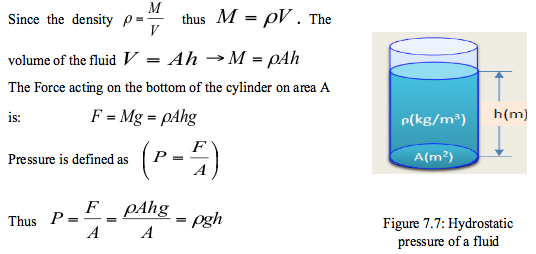
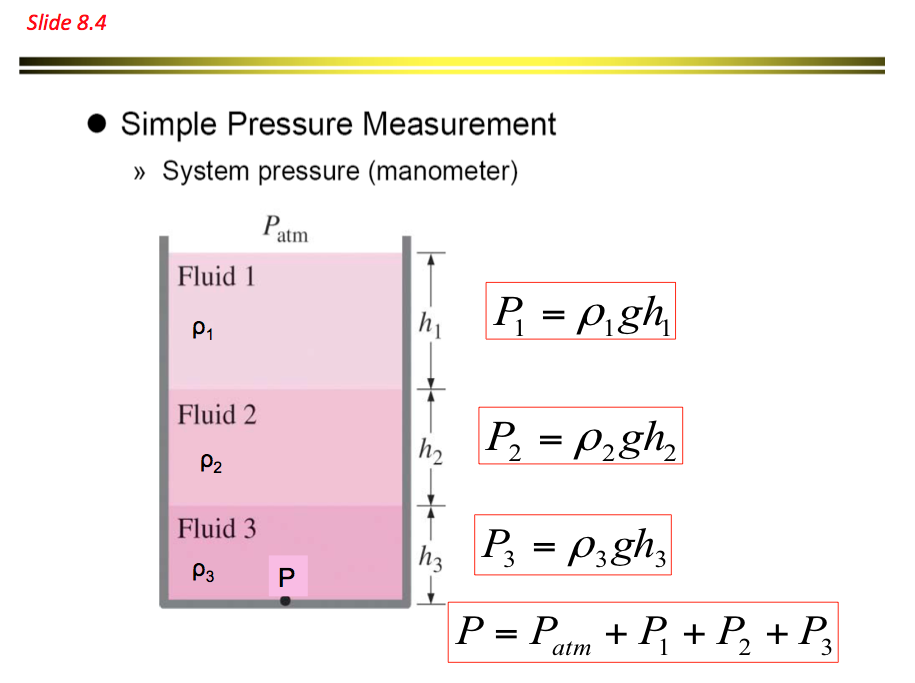
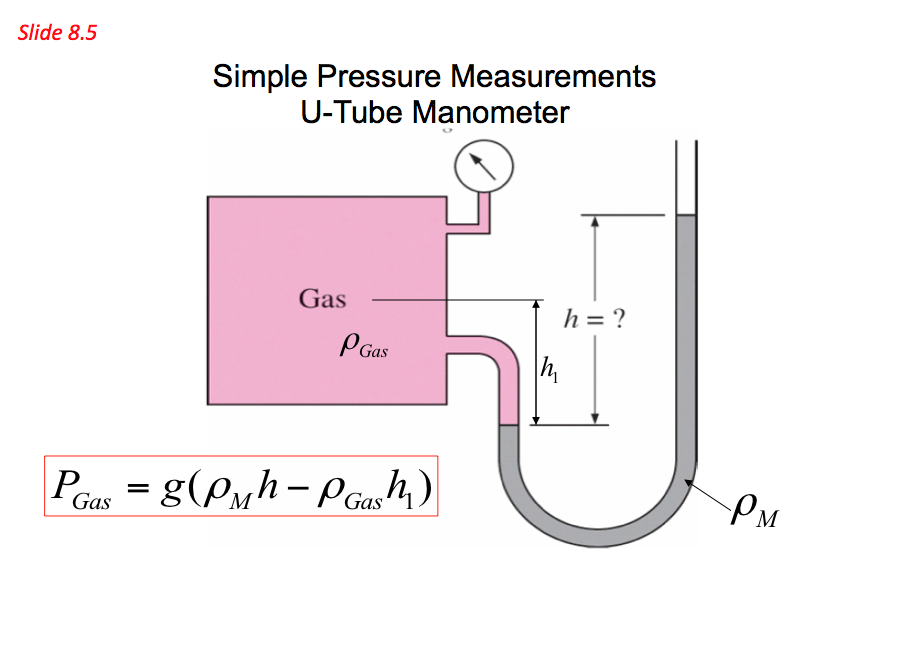
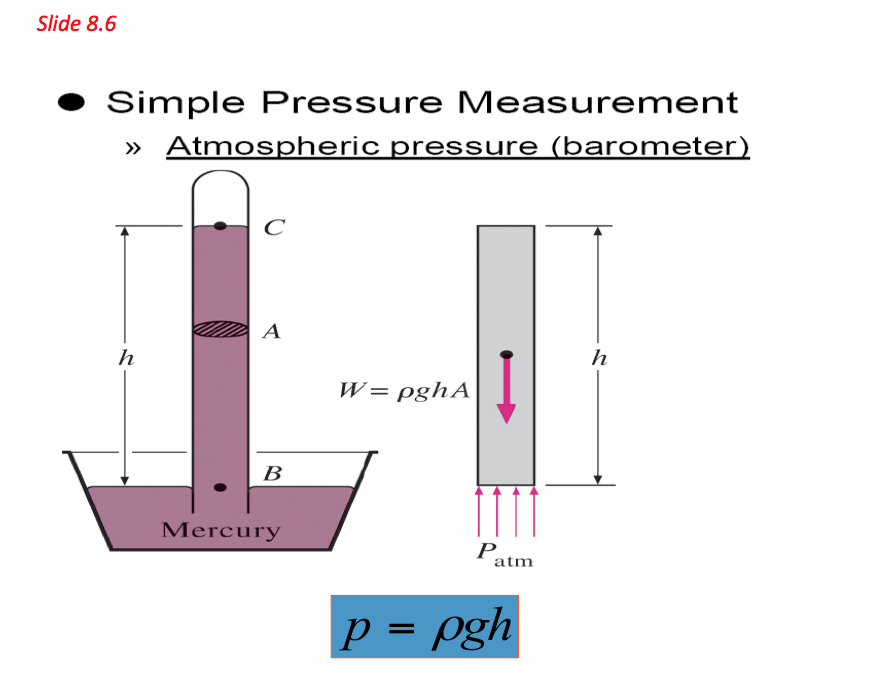
- The above equation indicates that a fluid column can be used to measure pressure differences. Based on these principles a device called monomer is established and is commonly used to measure pressure differences. Pressures measured with barometers such as Bourdon tube gage and manometers are referred to as gauge pressure
VERY IMPORTANT: In thermodynamic relationships, absolute pressure is always used unless specifically stated otherwise.
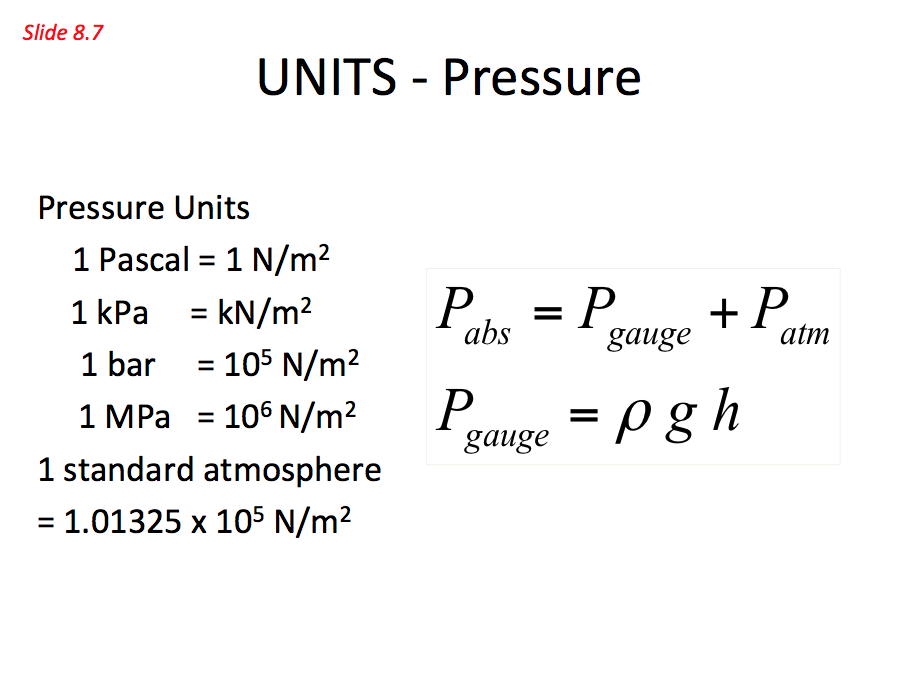
Absolute pressure = Guage pressure + Atmospheric pressure
Pabs = Pguage + Patm where Pguage = pgh
- Properties and Units 📷
- Pressure 📷
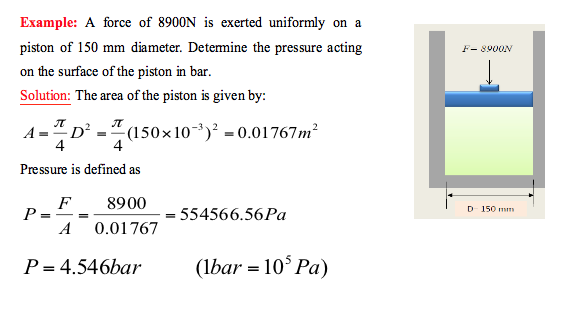
- Example: Convert 776mmHg to MPa
- Solution: Hg is the chemical symbol for mercury and the density of mercury is
Pmercury = 13.6x103kg/m3 and g=9.81m/s2
h=776mm=776x10-3m
P = pgh = (13.6x103)(9.81)(776x10-3) = 103530.816Pa = 0.10353MPa
Important note: the topic of manometers is comprehensively covered in the first part of Mechanical principles B module under hydrostatic pressure.
Simple Pressure Measurement 📷
Simple Pressure Measurement - U-Tube Manometer 📷
Simple Pressure Measurement - Atmospheric Pressure Barometer 📷
Units - Pressure 📷
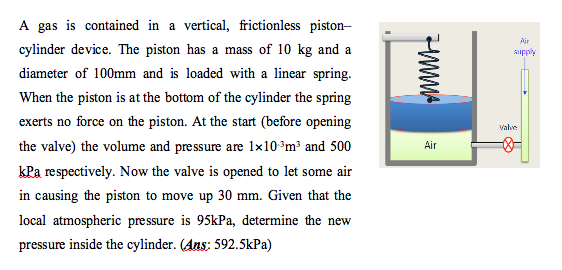
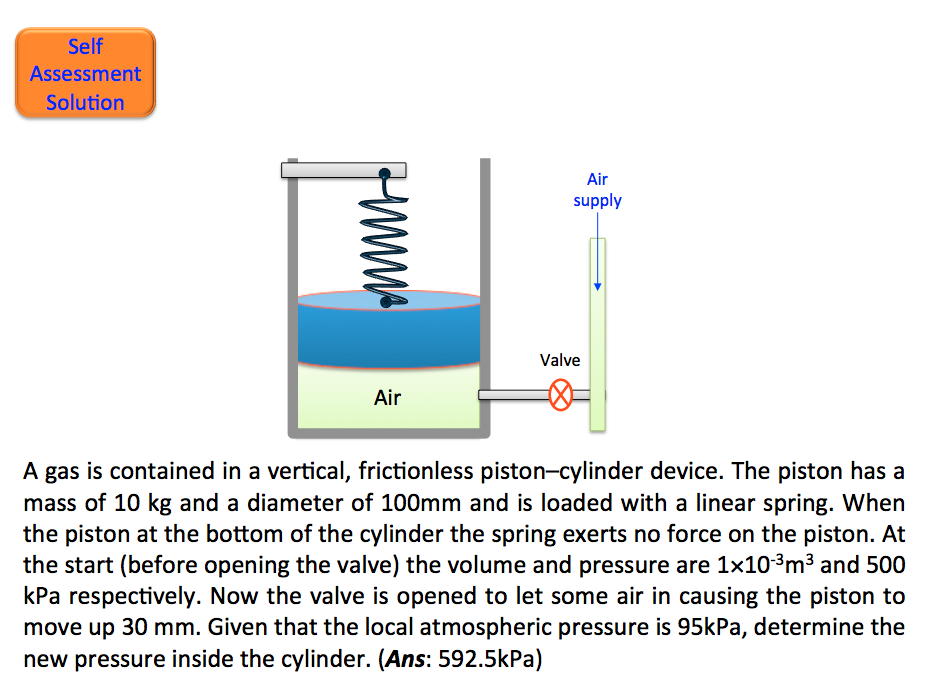
- Self Assessment Question
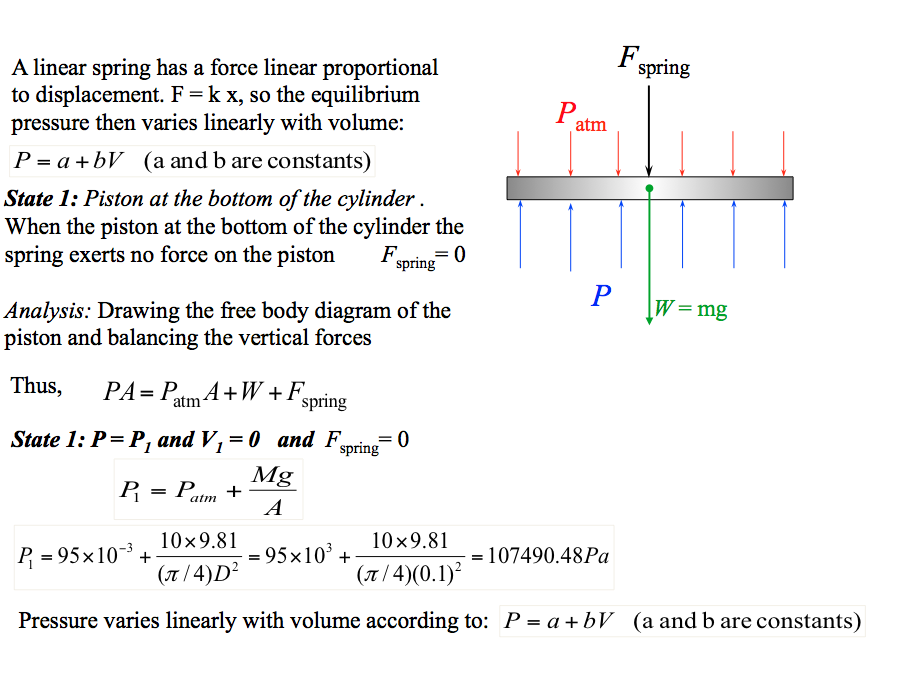
- Self Assessment Solution 📷

- Pressure is defined as the amount of perpendicular force (F) exerted by a fluid on a unit surface area (A). The term pressure is used for fluid and normal stress for solids. As stated in the definition above, pressure is force per unit area:
-
8.3 Specific volume (v) and density (ρ)
- Volume is a property associated with the cubic mass of a substance.
Although the SI unit for volume is the cubic meter, a commonly used volume unit is the litre (L) and 1 L = 10-3 m3. An important measurable quantity in engineering thermodynamics is the specific volume and is defined as the volume per unit mass of substance and is given the symbol v ( ). Specific volume is the reciprocal of the density (ρ) i.e. . Specific volume and density are intensive properties and may vary from point to point. The SI unit for specific volume and density are m3/kg and kg/m3 respectively. - Volume is a property associated with the cubic mass of a substance.
-
Self Assessment Questions
- 1. One watt is equal to
a. 1 Nm/s
b. 1 N/min
c. 1 Nm/hr
d. 1 kNm/hr
e. 1 kNm/min.
2. A system contains 1.45 kg of gas with a specific enthalpy of 1200 kJ/kg. The total system enthalpy is
a. -1200kJ
b. +1200kJ
c. -1740kJ
d. +1740kJ
3. A system contains 1.45 kg of gas occupies a volume of 0.87 m³, the specific volume is
a. 0.600 m³/kg
b. 0.600 m³
c. 0.600 kg/m³
d. 1.6kg/m3
4. A system contains 1.45 kg of gas occupies a volume of 0.87 m³, the gas density is
a. 0.600 m³/kg
b. 1.45kg/m3
c. 0.600 kg/m³
d. 1.667 kg/m³
Correct Answers
- 1. One watt is equal to
a. 1 Nm/s
b. 1 N/min
c. 1 Nm/hr
d. 1 kNm/hr
e. 1 kNm/min.
2. A system contains 1.45 kg of gas with a specific enthalpy of 1200 kJ/kg. The total system enthalpy is
a. -1200kJ
b. +1200kJ
c. -1740kJ
d. +1740kJ
3. A system contains 1.45 kg of gas occupies a volume of 0.87 m³, the specific volume is
a. 0.600 m³/kg
b. 0.600 m³
c. 0.600 kg/m³
d. 1.6kg/m3
4. A system contains 1.45 kg of gas occupies a volume of 0.87 m³, the gas density is
a. 0.600 m³/kg
b. 1.45kg/m3
c. 0.600 kg/m³
d. 1.667 kg/m³
- 1. One watt is equal to
-
Thermodynamics Tutorial 8
- 1. The height of a column of mercury, ρ=13.6×103 kg/m3, that will exert a pressure equal to the atmosphere is:
a. 1.00 m
b. 12.2 mm
c. 76m
d. 760 mm
2. A pressure that will support a column of mercury (Hg) to a height of 256 mm would support a column of water to what height? The density of mercury is 13.6×103 kg/m3; the density of water is 1000 kg/m3. Choose the correct answer form the following:
e. 3.482 m.
f. 1.00 m
g. 18.8 mm
h. 33.8 m
i. 760 mm
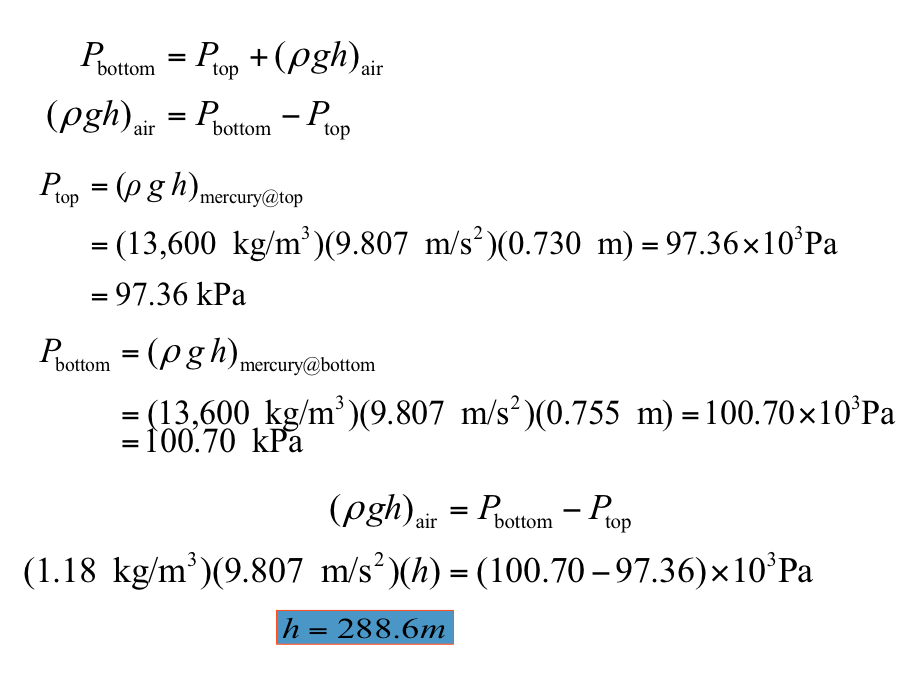
3. The basic barometer can be used to measure the height of a building. If the barometric readings at the top and at the bottom of a building are 730 and 755 mm Hg, respectively, determine the height of the building. Take g =9.807m/s2 and the densities of air and mercury to be 1.18 kg/m3 and 13,600 kg/m3, respectively. (Ans: h = 288.6 m)
Click this to see the solution 📷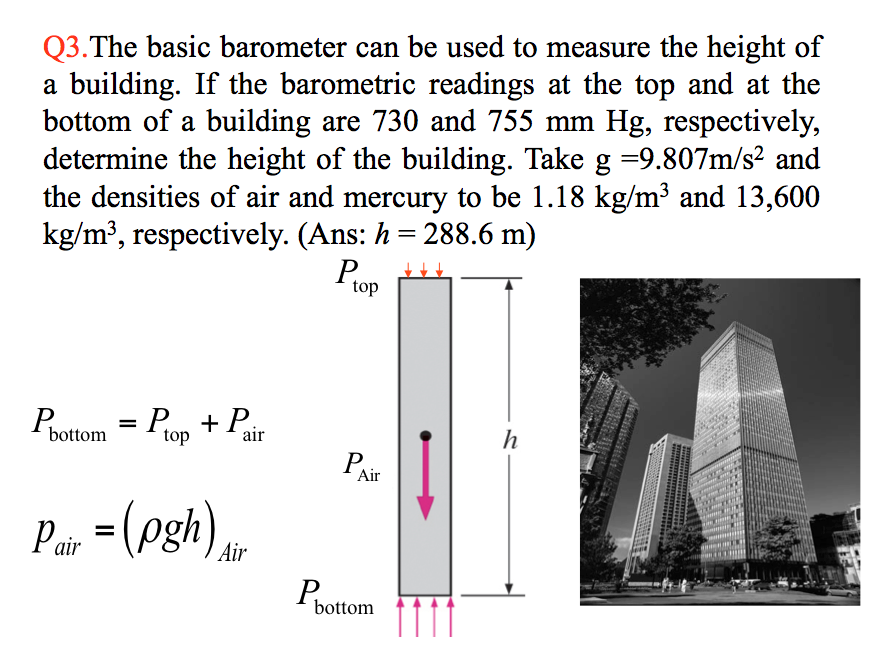
- 1. The height of a column of mercury, ρ=13.6×103 kg/m3, that will exert a pressure equal to the atmosphere is: