06
-
- Introduction
- This week we start our look at “real” materials – polymers. We will look at the underlying carbon chemistry.
- Study Plan
- Study:
1. Read over week 6 lecture presentation
2. Read chapter 14.1-14.4 and 15.20 in Callister
3. Work through the questions 1 to 3 in the Polymer chemistry tutorial A below and other relevant questions at the end of the chapter in Callister
Activity:
Look at the videos referenced in the study pack
Assessment:
Class Test 1 -
- Introduction to carbon chemistry I
- Carbon is to be found in group IV of the periodic table i.e carbon has a valency of 4. Instead of losing these "spare”electrons to form C4+ or gain another electron to form C4-carbon prefers to SHARE electrons with other atoms, e.g.
C C ---> C--C
i.e. single bond
This process can continue leading to long chains of carbon atoms i.e., C--C--C--C--C--C--C--C--C--C--C--C--C--C--C--C
But considering that carbon forms 4 bonds around itself then by the rules of Valence Shell Electron Pair Repulsion the shape of the molecule will be Tetrahedral i.e.
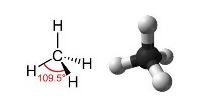
With the carbon atom sitting in the middle of the tetrahedron. This implies that the representation of the carbon chain shown above should in fact be represented as follows,
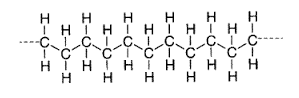
This is further shortened to the following,
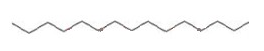
At room temperature, the C--C bond allows rotation about itself.
For example,
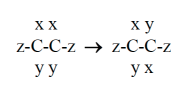
This is NOT the case with carbon-carbon double bonds. These are formed where two electrons from each carbon atom are used in the sharing process i.e.
C C = C=C
This molecule is planer in shape.
The C=C bond is very reactive i.e. it will react to form C-C bonds e.g.
-C=C + C=C- "" -C-C-C-C-
New bond formed therefore increasing chain length.
This bond also "locks” the molecule in shape due to its shape and inability to rotate e.g.
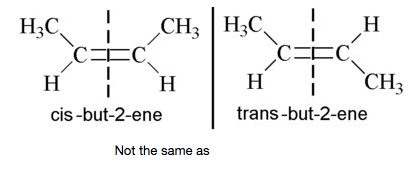
These are two different compounds with different properties. So it can be seen that the presence of a double bond within a chain imparts two main properties to the chain.
1) It "stiffens " the chain.
2) Allows the chain to undergo further growth via double bond cleavage and reformation of single bonds
Organic Chemistry video -
- Polymerisation
- This is the process by which small molecular units called monomers react together to form long chains i.e. polymers.
There are a number of ways in which this process can take place of which two are predominant,
1. ADDITION POLYMERISATION
This was described earlier and as the name suggests occurs by the addition of more monomer units together i.e.
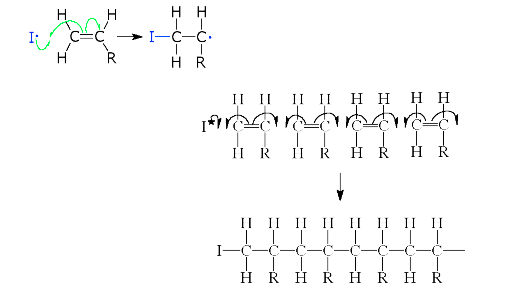
One electron pair from the double bond moves to the other side of the atom and "pairs up” with an equivalent electron from the other unit to form a new bond. The single electron at each end of the dimer is now available to form new single bonds with other monomer units.
Some examples of this type of polymerisation are:
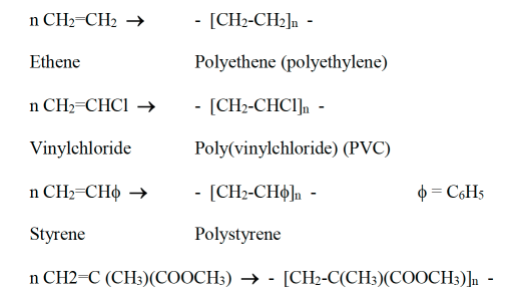
Methylmethacrylate Poly(methylmethacrylate) (PMMA) (PERSPEX) (Have a look at this site)
2. CONDENSATION POLYMERISATION
This type of polymerisation occurs by chemical reactions between two ends of a molecule with the elimination of by-products usually water.
e.g.
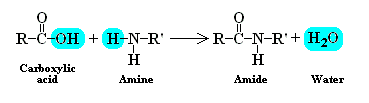
This reaction would stop as soon as the reaction is complete since there are no free reaction centres left. However, if each molecule had a reaction centre at each end then the reaction would proceed until all of the reactants were used up and the chain would grow There are two possibilities:
a) the molecule has the same reaction centres at each end, e.g.
HO-R’-OH (1)
therefore another molecule is required to complete the reaction scheme i.e.
HOOC-R’’-COOH (2)
If (1) & (2) react, they would produce ;
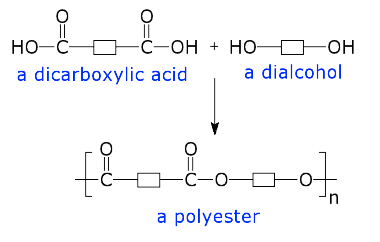
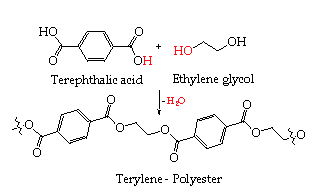
An example of this is
Another example would be the reaction of Adipic acid (HOOC-(CH2)4–-COOH) and Hexamethylene diamine (H2N-(CH2)6–-NH2)
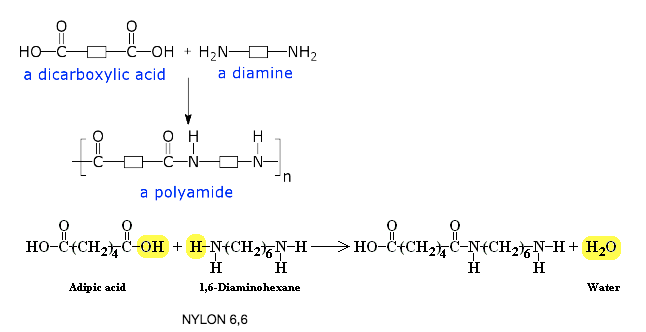
(View Nylon Rope trick)
(b) The molecule has a different reaction centre at the molecule e.g H2N-R’-COOH
This implies that only one type of molecule is required in the polymerisation process.
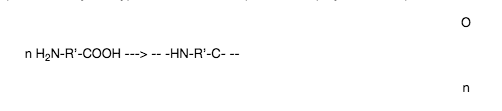
an example of this is the polymerisation of 6-aminoheptanoic acid,
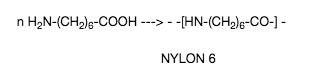
Polymerization video -
-
- POLYMER CHEMISTRY TUTORIAL
- 1. What is the major significance of Carbon having a valency of 4 ? (answer)
2. Explain the difference in structure and properties between the first 10 Alkanes, C20H22, C100H102 and C20,000 (answer).
3. Describe using suitable examples the two main polymerization methods used in theproduction of polymers. (answer in pages 49-51)