07
-
- Introduction
- This week we continue our study of polymers. We will look at the different types of bonding evident in polymers.
- Study Plan
Study:
1. Read over week 7 lecture presentation
2. Read chapter 2.7 in Callister
3. Work through the questions 1 and 2 in the Polymer chemistry tutorial B below and other relevant questions at the end of the chapter in Callister
Activity:
1. Look at the videos referenced in the study pack
2. View the VMSE on Repeat Unit and Polymer Structures -
- Polymer chemistry II
- INTRODUCTION TO CARBON CHEMISTRY II (BONDING) In carbon chemistry, there are two main types of bond:
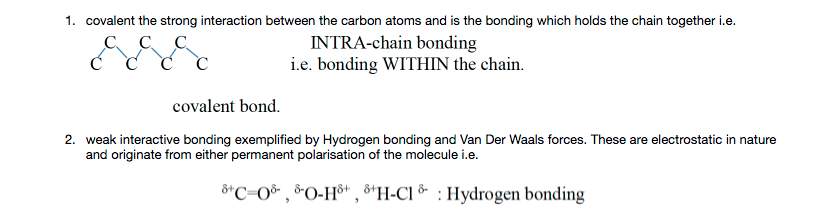
or instantaneous or momentary polarisation i.e one end of the molecule has a little more electron density around it and hence obtains a slight negative charge (Van Der Waals force) e.g.
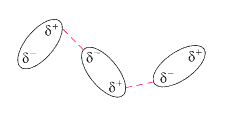
This can then multiply to produce a weak interaction between the molecules. This type of bonding occurs in ALL atomic and molecular-covalent materials.
It is this type of bonding which holds the polymer chain together, i.e.
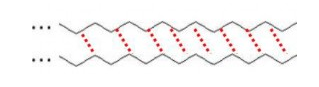
INTER-chain bonding bonding BETWEEN the chains. weak inter-chain bonding.
However, it is also possible to get STRONG interchain bonding by allowing the chains to have COVALENT bonds between them.
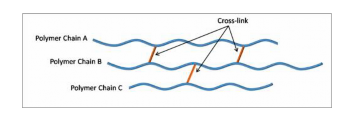
This is termed CROSS-LINKING and imparts a high degree of rigidity to the polymer chain. The significance of these types of bonding will be discussed in greater detail as we progress through the course.
Have a look at this video -
- Polymer types
- There are two main types of polymers : 1. Thermoplastic
2. Thermosetting
The major difference between these two types of polymers is the form of interchain bonding.
Thermoplastic
In This type of polymer the interchain bonding is that of Van Der Waals forces. As polymerisation proceeds the molecules reach a size where the Van Der Waals forces become effective so that the liquid becomes relatively viscous.
As polymerisation proceeds the average chain increases resulting in an increase in viscosity due to a greater and greater degree of chain entanglement. As polymerisation nears completion the chains become very long so that the sum of the Van Der Waals forces acting between adjacent molecules becomes considerable and the material is so viscous as to be regarded as a solid. It no longer flows unless it is heated. This increases the energy of the molecules so that they are able to overcome the Van Der Waals forces acting between them. Such a substance is called a THERMOPLASTIC, since it can be softened repeatedly by the application of heat. This is not the case with THERMOSETTING polymers.
Thermosetting
These are plastic in the primary stages of manufacture but once moulded to shape they "set" and cannot subsequently be softened by re-heating. This is because a permanent covalent bond is formed between the chains.
It can therefor be seen that the type of bonding which exists between the individual chains affects the ultimate mechanical properties of the material contrast the % elongation of thermoplastics with that of thermosetting polymers. -
-
- Polymer Chemistry Tutorial
1. Discuss the affects which secondary bonding has on the properties of polymers. (answer)
2. Explain the differences between Thermoplastic and Thermoset polymers.