08
-
- Introduction
- This week we start our look at the physical properties of polymers, and examine how the chemical structure effects these properties.
- Study Plan
- Study:
1. Read over week 8 lecture presentation
2. Read chapter 14.6-14.11 in Callister
3. Work through questions 6 in the Polymer chemistry tutorial below and other relevant questions at the end of the chapter in Callister
Activity:
Look at the videos referenced in the study pack -
- Physical nature of solid polymers
- So far we have looked at how polymer chains are built up from monomer units and the effect of different forms of interchain bonding. We will now move on to look at the physical nature of the polymer in the solid state.
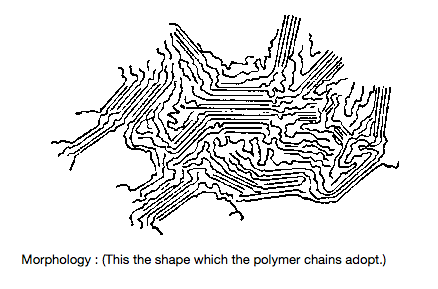
The physical nature of the solid polymer will depend to a large extent upon its chemical nature There are two main forms which a polymer can adopt:
Crystalline : in which the chains are close together and are arranged in an ordered structure. Amorphous : the chains are randomly arranged with no ordered structure.
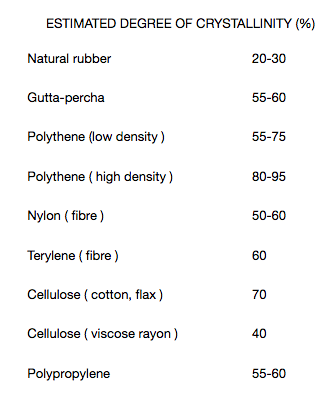
In practice the polymer will contain "islands” of crystallinity. The exact proportions of which will vary from polymer to polymer. Some polymers are more crystalline than others due to the difference in the nature of the side-groups, e.g -H, -CH3, C6H5– -COOR, and on the number of such side-groups For example :
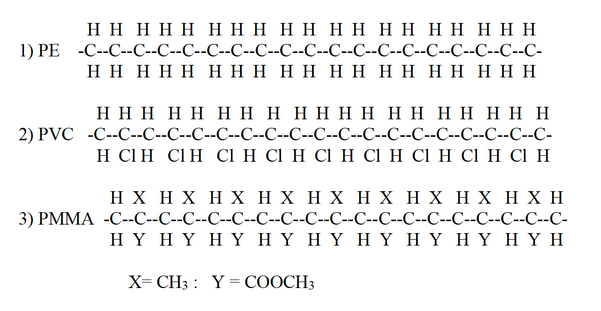
These side-groups do not allow the polymer chains to inter-mesh together very well This is due to an effect called STERIC HINDRANCE where two groups of atoms are in close proximity there will be an interaction between the two due to their size and shape This effect increases with increasing size and shape. For example Polystrene:
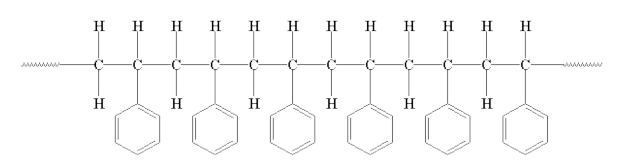
Hence it can be seen that polymers with large substituent groups will be less crystalline than those with small substituent groups.
We will now discuss these forms in a little more detail.
● see The crystalline state -
- The crystalline state
- When polymers are irradiated by a beam of X-rays scattering produces diffuse haloes on the photographic plate for some polymers while for others a series of sharply defined rings superimposed on a diffuse background is recorded. The former are characteristic of amorphous polymers and illustrate that a limited amount of short range order exists in most polymeric solids. The latter patterns are indicative of considerable three-dimensional order and are typical of polycrystalline samples containing a large number of unorientated crystallites associated with amorphous regions.
The two main factors which affect the degree of crystallisation in polymers are:
1. the size and shape of the side-groups in the polymer as mentioned earlier. 2. the type and strength of intermolecular bonding which is dependent upon (i) above.
Intermolecular bonding In polyethylene crystallites¬ the close packing achieved by the chains allows the van der waals forces to act co-operatively and provide additional stability to the crystallite. Polymers containing polar groups e.g Cl, CN, OH, can be held rigid and aligned in a polymer matrix by the strong dipole-dipole interactions between the substituents,
e.g Nylon 6,6 which has a Tm of 540 K compared with 410 K for Polyethylene.
-
-
- Polymer Chemistry tutorial
- POLYMER CHEMISTRY TUTORIAL
1. What is the major significance of Carbon having a valency of 4 ?(answer)
2. Explain the difference in structure and properties between the first 10 Alkanes, C20H22, C100H102 and C20,000 (answer)
3. Describe using suitable examples the two main polymerization methods used in the production of polymers. (answer in pages 49-51)
4. Discuss the affects which secondary bonding has on the properties of polymers. (answer)
5. Explain the differences between Thermoplastic and Thermoset polymers.
6. Describe fully the ideas behind crystallization in polymers and explain the main factors affecting the extent of crystallization (answer)
7. Discuss fully the five regions of viscoelasticity. Define the terms Tg and Tm