09
-
- Introduction
This week we continue our look at the physical properties of polymers, and examine how the mechanical properties of polymers can be explained.
- Study Plan
Study:
1. Read over week 9 lecture presentation
2. Read chapter 15.1- 15.8, 15.12-14 in Callister
3. Work through questions 7 in the Polymer chemistry tutorial below and other relevant questions at the end of the chapter in Callister
Activity: Look at the videos referenced in the study pack -
- The amorphous state
- In the amorphous state the distribution of polymer chains in the matrix is completely random with none of the structures imposed by the ordering encountered in the crystallites of partially crystalline polymers. This allows the onset of molecular motion in amorphous polymers to take place at temperatures below the melting temperatures of such crystallites. Consequently as the molecular motion in an amorphous polymer increases the sample passes from a glass through a rubber-like state until finally it becomes molten These transitions lead to changes in the physical properties and material application of a polymer and it is important to examine physical changes in an amorphous polymer as a result of variations in the molecular motion.
The five regions of viscoelastic behaviour :
The physical nature of an amorphous polymer is related to the extent of the molecular motion in the sample, which in turn is governed by the chain flexibility, and the temperature of the system.
There are five distinct regions of structural effect these are shown on the graph below:
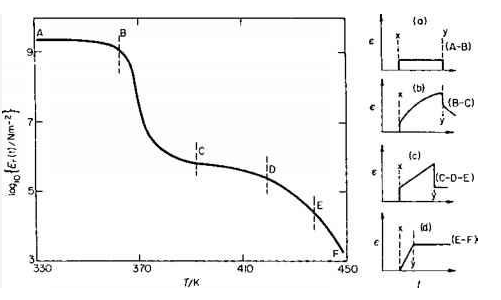
1. THE GLASSY STATE This shown by section A-B on the graph i.e that part of the graph lying below 363 K. At this temperature all molecular chain motion is frozen Thus causing the material to act in an elastic manner as shown in (a)
relaxation modulus Er = 109.5 – 109 Nm-2
2. LEATHERY STATE This is the transition region denoted by B-C (temperature range 363-393 K). With an increase in temperature the polymer chains begin to "unfreeze” and are thus able to partially move. The temperature at which This begins to happen is called THE GLASS TRANSITION TEMPERATURE denoted by Tg Just above Tg the movement of the polymer chain segments is still quite slow. The strain timecurve is shown in (b)
Er = 105.7 Nm-2
3. RUBBERY STATE At approximately Tg + 30 K the curve begins to flatten out into a plateau region C-D¬ This extends up to 420 K.
Er = 105.7 - 105.4 Nm-2
4. RUBBERY FLOW region D-E in the graph. In which elastic flow is observed to occur. The effect of the applied stress in states iii and iv is shown in (c).
Er = 105.4 - 104.5 Nm-2
5. VISCOUS STATE At this point in the curve E-F the temperature is such that the polymer chains are free to move (This can also be defined as the "melting temperature Tm of the polymer) and as such act like a viscose liquid . Here Er decreases steadily with temperature from 104.5 Nm-2
- Glass transition and melting temperatures for some polymers
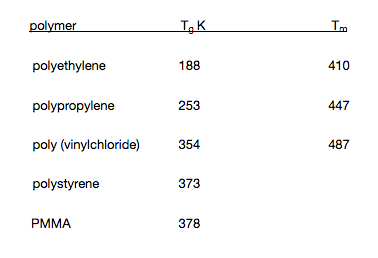
It can be seen that the nature of the side-group determines to a certain extent the Tg of the polymer.
(Overview) (review) -
-
- Polymer Chemistry tutorial
- POLYMER CHEMISTRY TUTORIAL
1. What is the major significance of Carbon having a valency of 4 ?(answer)
2. Explain the difference in structure and properties between the first 10 Alkanes, C20H22, C100H102 and C20,000 (answer)
3. Describe using suitable examples the two main polymerization methods used in the production of polymers. (answer in pages 49-51)
4. Discuss the affects which secondary bonding has on the properties of polymers. (answer)
5. Explain the differences between Thermoplastic and Thermoset polymers.
6. Describe fully the ideas behind crystallization in polymers and explain the main factors affecting the extent of crystallization (answer)
7. Discuss fully the five regions of viscoelasticity. Define the terms Tg and Tm