01
-
Introduction
- I would like to welcome you to this module on Materials Engineering. In this module we will be looking at materials, what they are, how the act and their properties. No previous knowledge of chemistry is required as I will fill you in with the main ideas. I hope that you enjoy the module and get a lot from it. This study pack will form the core of your learning material. You will see in the notes that I have included hyperlinks to external support material in the form of videos, notes etc. As well as this, the core textbook comes with access to the VMSE resource area. I would suggest that you access this through Mozilla Firefox with Java and Flash add-ins enabled. I would encourage you to use them.
- Week 1
Objective This week we will be looking at the world of materials and why Engineers should be interested in it. Study 1. Read over week 1. Intro, the two lecture presentations and the references.
2. Read chapter 1 & 2.1 in Callister.Activity Undertake activity in student note week 1. Resources - Book Website VMSE
-
1. BASIC STRUCTURE OF MATERIALS
- All matter is made up of atoms. Atoms are very tiny particles indeed (10-10m radius). A pin head contains about 3.5 x 1017 of them - give or take a few million. Altogether there are ninety two different types of atoms which occur naturally though during the ‘nuclear age’ scientists have succeeded in producing some new ones, for example, plutonium. Of the naturally occurring atoms the smallest and simplest is that of hydrogen; whilst the largest, some two hundred and thirty eight times as massive, is that of uranium.
A chemical element contains atoms all of one type. Out of all the elements over seventy are metals. Some of these metallic elements are extremely rare, whilst others are useless to the engineer either by virtue of poor mechanical properties or of chemical instability. Consequently, less than twenty of them are in common use in engineering alloys.
Of the non-metallic elements carbon is perhaps the one which forms the basis of most engineering materials since it constitutes the ‘backbones’ of all plastics. Moreover, it can be used in strong fibre form and is an essential constituent of all common heat-treatable steels. Chemically similar to a member of the same chemical ‘family’ - the element silicon has become famous in the form of the ‘silicon chip’, but along with oxygen (as silicon dioxide or silica) it is the basis of may refectory building materials. Oxygen and silicon are by far the most common elements in the Earth’s crust and account for some 75% of it in the form of clays, sands, stones and rocks like granite.
All solid materials consist of atoms which are arranged in some pattern peculiar to that material. These atoms are held together by forces of attraction which have their origin in electrical charges within each atom. - All matter is made up of atoms. Atoms are very tiny particles indeed (10-10m radius). A pin head contains about 3.5 x 1017 of them - give or take a few million. Altogether there are ninety two different types of atoms which occur naturally though during the ‘nuclear age’ scientists have succeeded in producing some new ones, for example, plutonium. Of the naturally occurring atoms the smallest and simplest is that of hydrogen; whilst the largest, some two hundred and thirty eight times as massive, is that of uranium.
-
1.1 Atomic Structure
- An atom consists of several sub-atomic particles. The main sub-atomic particles are:
• electron;
• proton;
• neutron.
The protons and neutrons are concentrated in the core - or nucleus - of the atom, whilst the electrons are arranged in a cloud around the nucleus. The proton and the neutron are roughly equal in mass (1.67 x 10-27kg) whilst the electron is only about one two thousandth of the mass of the other two (9.11 x 10-27). However, it is the electrical charges carried by these particles which are more important. The electron carries a unit charge (1.6 x 10-19 coulombs) negative electricity, whilst the proton carries an equal but opposite charge of positive electricity. The neutron, as its name suggests carries no resultant electrical charge and in all atoms other than the radioactive ones the neutron can be regarded as ‘dead weight’ in the nucleus. It affects only the ‘atomic weight’ or more properly, the mass of the element. Atomic mass (A) of a specific atom may be expressed as the sum of masses of protons and neutrons within the nucleus.
Each chemical element is characterized by the number of protons in the nucleus, or the atomic number (Z). For an electrically neutral or complete atom, the atomic number also equals the number of electrons. This atomic number ranges in integral units from 1 for Hydrogen to 103 for Lawrencium. - An atom consists of several sub-atomic particles. The main sub-atomic particles are:
-
Electron Configurations
- Electrons surrounding the atomic nucleus are not at the same energy level. Consequently, it is convenient to group electrons into shells with different energy characteristics. The first shell, nearest to the nucleus, contains only two electrons, the second eight, the third eighteen, and so on. The greatest number of electrons in a given shell is 2n2 where n is known as the quantum number of the shell. Each shell is designated a letter. The shells can be subdivided into subshells.
Fig. 1 shows the number of electron states in some of the electron shells and subshells.
Fig.2 shows the relative energies of the electrons for the various shells and subshells.
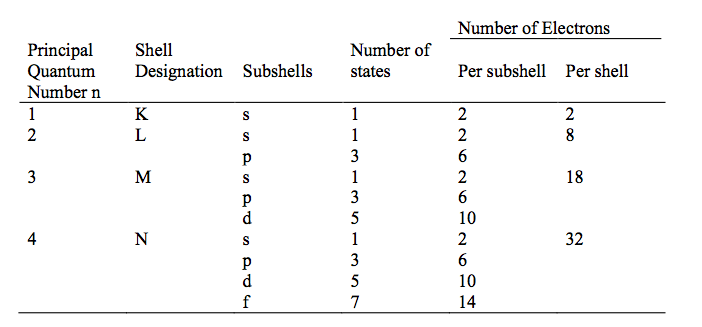
- Figure 1: The Number of Available Electron States in Some of the Electron Shells and Subshells.
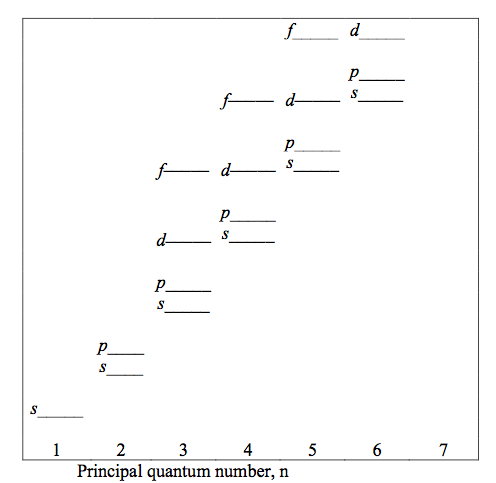
- Figure 2: Schematic representation of the relative energies of the electrons for the various shells and subshells.
- Electrons surrounding the atomic nucleus are not at the same energy level. Consequently, it is convenient to group electrons into shells with different energy characteristics. The first shell, nearest to the nucleus, contains only two electrons, the second eight, the third eighteen, and so on. The greatest number of electrons in a given shell is 2n2 where n is known as the quantum number of the shell. Each shell is designated a letter. The shells can be subdivided into subshells.
-
ATOMIC STRUCTURE/PERIODIC TABLE/BONDING: LECTURE SUMMARY
1. The atom is made up of three fundamental particles:
the proton
the neutron
and the electron.
The proton and the neutron are found in the nucleus which has a density of 1017 Kgm-3 and a diameter of approximately 10-15m. The electron is very small and has negligible mass relative to the other two particles.2. It is the arrangement of the electrons in the atom which determines the chemical and physical behaviour of the aton and element. The electrons in an atom can be considered to orbit around the nucleus in regular "shells" like the planets around the sun. The maximun number of electrons in a particular shell is given as 2n2 (where n is the shell number, i.e. first, second etc) 3. The grouping of elements according the number of electrons in the outermost shell (valence shell) and hence their chemical properties is called the periodic table of the elements. 4. The main way in which atoms combine together is called BONDING. There are four main types of primary bonding:
IONIC :complete transfer of electrons;
COVALENT: sharing of electrons
POLAR COVALENT: incomplete sharing of electrons;
METALLIC: "sea of electrons".
and three types of secondary bonding:
VAN DER WAALS;
DIPOLE-DIPOLE;
HYDROGEN.Reference 1) Properties of Engineering Materials, R.A. Higgens, pp 1-9,18- 29.